Corporate Headquarters:
Bimini Health Tech
8400 Belleview Drive, Suite 125
Plano, TX 75024
+1 (858) 348-8050
Research & Development:
Bimini Health Tech
420 Stevens Ave, Suite 220
Solana Beach, CA 92075
+1 (858) 348-8050
Serene Structured Saline Breast Implant

Uniquely designed saline implant with an internal structure.
Thoughtfully engineered to provide a more customized fit based on the patient’s desired needs and outcomes.
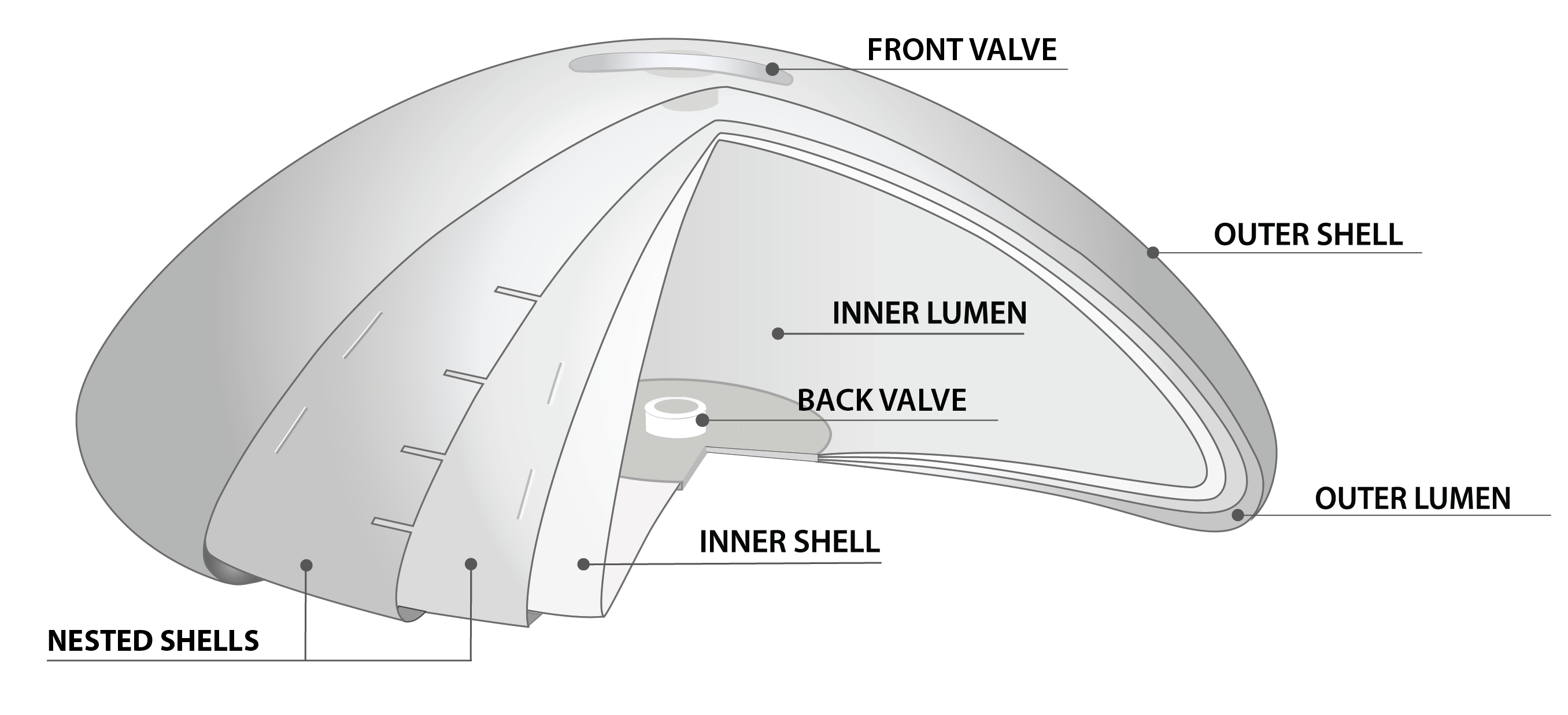
What makes the Puregraft Serene Structured Breast Implant different?

Dual Fill Chambers Ensure: A stable implant base, adjustable height and projection and upper pole fullness.
Interior Shells: Control the movement of saline, which helps minimize edge collapse, rippling and wrinkling.
Made in the U.S.A: Generation 2 technology utilizes a robotic dipping method which creates a more uniform implant shell.
No MRI or Scans required to detect implant rupture.
Pure Confidence Warranty: Provides lifetime coverage for implant replacement due to deflation or rupture.

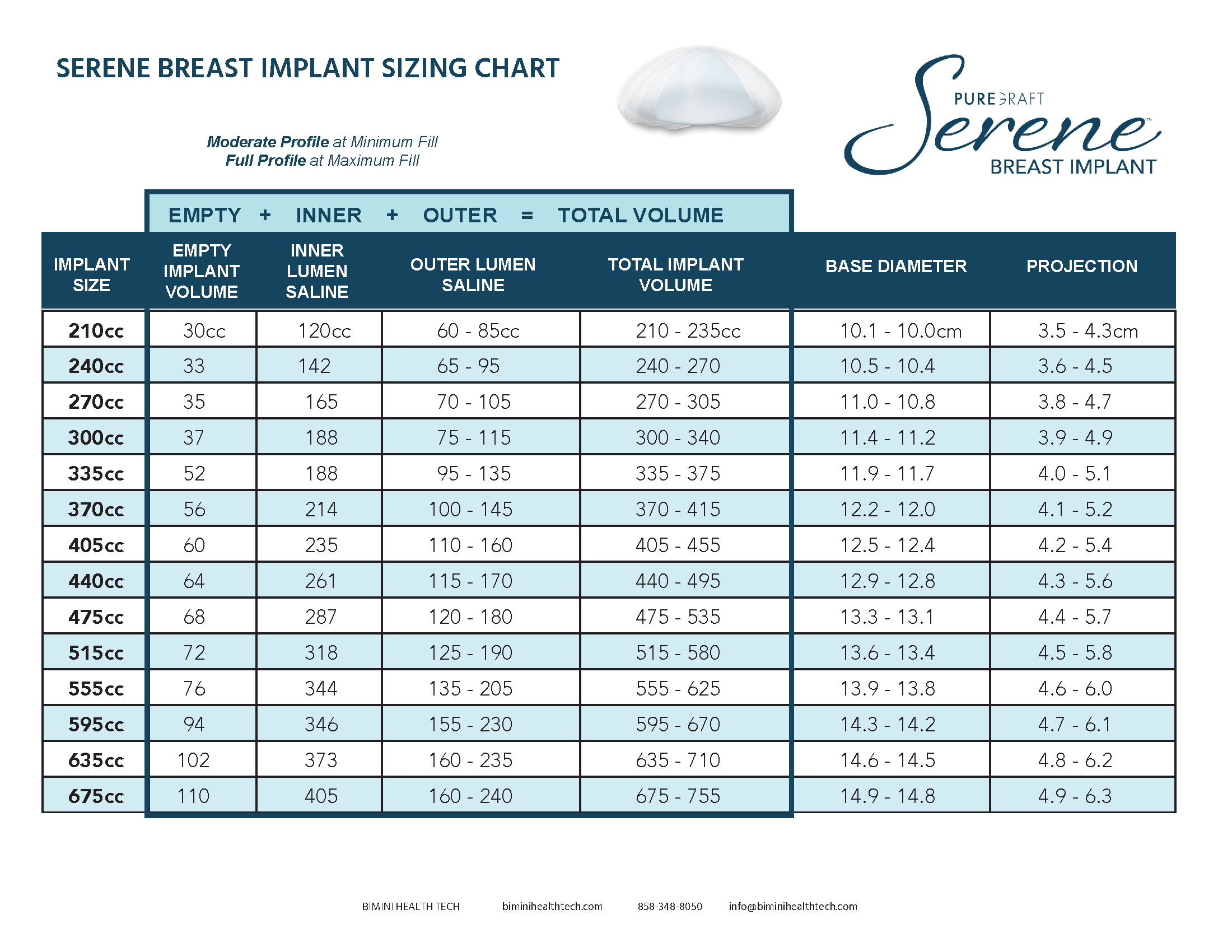
Serene Structured Saline Breast Implant is available in a range of sizes providing options for projection & height
Resources
Collateral

Reporting Forms
Forms are for Puregraft Serene Breast Implants (formerly Ideal Implant)